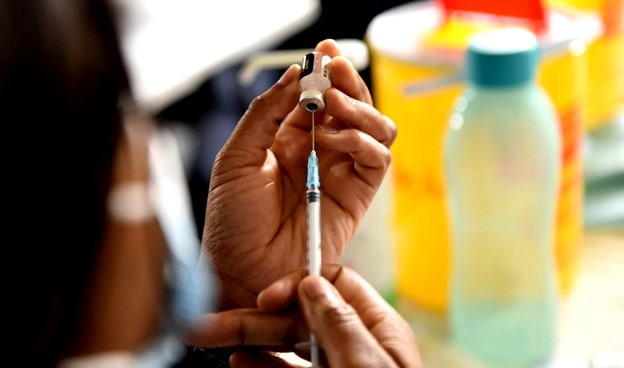
Arguably, the biggest HIV news of the year so far broke at the end of June when it was reported that an injection containing the antiretroviral drug Lenacapavir provides young women and adolescents with six months of highly effective protection against HIV infection per jab.
Dr Nkosiphile Ndlovu, a senior research clinician in the Clinical Trials Division of the Wits Reproductive Health and HIV Institute Research Centre, pointed out that there are two registrational trials looking at the Lenacapavir injection for HIV prevention. The one in young women and adolescents that was just reported is called PURPOSE 1. PURPOSE 2, a similar study conducted in men, transgender people and gender non-binary individuals who have sex with men, is ongoing. Ndlovu was involved in PURPOSE 1.
Mitchell Warren, executive director of a US-based HIV advocacy group, the Aids Vaccine Advocacy Coalition, told Spotlight results from PURPOSE 2 are expected late in 2024 or early in 2025. If these results show that Lenacapavir as injectable HIV prevention given every six months is safe and effective, Gilead will go to regulators and the World Health Organization (WHO) to have the product approved for use. In South Africa, Gilead will have to apply for registration with the South African Health Products Regulatory Authority.
But when and on what terms generic manufacturers will be allowed to produce the Lenacapavir jab remains uncertain.
Gilead previously told Spotlight it would follow a “direct voluntary licensing strategy for access to Lenacapavir in high-incidence, resource-limited countries”. We understand this to mean it will make licensing deals directly with generic companies, rather than through an intermediary such as the UN-backed Medicines Patent Pool (MPP). But Gilead is under substantial pressure to change course. Most recently, in a strongly worded statement, UNAIDS urged Gilead to work through the MPP.
Even if licenses are granted very quickly, directly or via the MPP, it is likely to take several years before generic companies can produce Lenacapavir jabs.